Why Does Metallic Character Decreases Across a Period
This is because the number of core electrons is the same therefore the shielding effect is constant. Ii On moving from the left to right in a Periodic Table metallic character decreases and non-metallic character increasesThis is because the capacity to lose electrons from outermost orbit of an atom called ionisation energy decreaseshenceamong the above elements Li being placed on extreme left side is most metallic and F being placed on extreme left side is most.
Metallic Character Of Transition Metals Transition Element
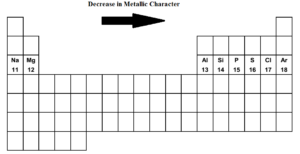
As metallic character decreases across a period left to right reactivity also decreases.

. For example Alkali metal group contains metals only. The energy levels are slowing. Not because its effective decreases morbidity mortality or diminishes any specific cancer rates.
However atomic radius decreases going across a period. The following general trends are observed as you go across period 3 from left to right. More protons are added but the outer valence shell remains the same so the positively charged nucleus draws in the electrons more tightly.
Thus we can conclude as we move left to right in a period the reactivity of elements gradually decreases up to group thirteen and then starts increasing. The nuclear positive charge increases. A The valency of an element is determined by the number of valence electrons present in the outermost shell of its atomThe number of electrons lost or gained or shared by one atom of an element to achieve the nearest inert gas electron configurationgives us the.
But for the nonmetallic elements the ionic radius increases because there are more electrons than protons. Thats the only reason chemotherapy is still used. F N C B.
Chemotherapy boosts cancer growth and long-term mortality. The number of valence electrons increases. Although reactivity of nonmetals increases on moving left to right across a period.
The attractive force on the valence electrons decreases as the atom gets larger. D Reactivity increases with atomic number in a group as well as in a period. Why does the ionization energy change when moving down a group of elements.
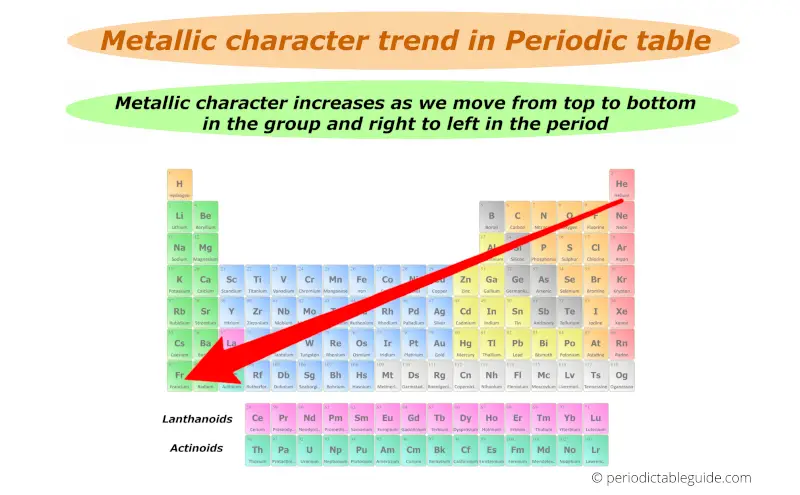
From right to left across a period metallic character increases because the attraction between valence electron and the nucleus is weaker enabling an easier loss of electrons. Metallic character tends to _____ down a group in the periodic table and _____ from left to right across a period. None of the above.
As we move across the period from left to right the non metallic character of elements increases. A Because all groups do not contain metals and non-metals. Melting points of these metals rise to a maximum at d5 except for anomalous values of Mn and Tc and fall regularly as the atomic number increases.
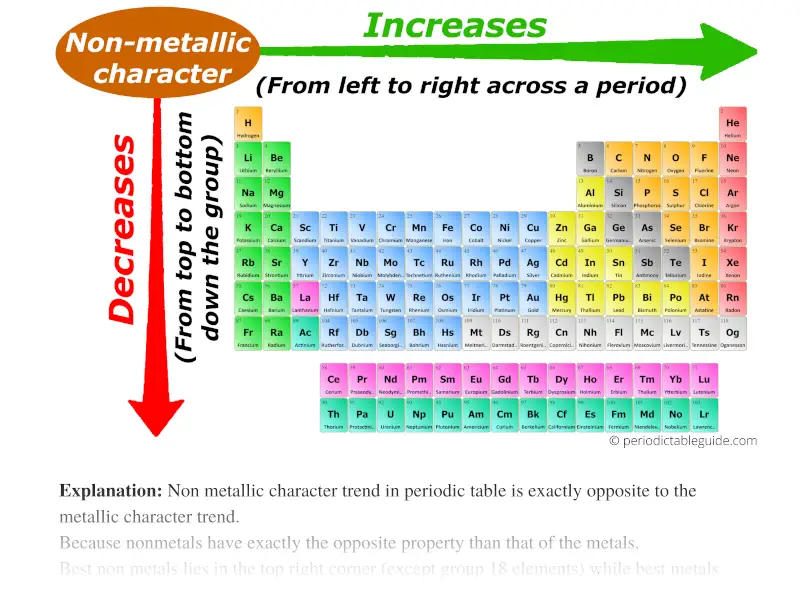
Non metallic character trend in periodic table is exactly opposite to the metallic character trend. The latest Lifestyle Daily Life news tips opinion and advice from The Sydney Morning Herald covering life and relationships beauty fashion health wellbeing. Metallic Character Boiling Point.
Free PDF download of NCERT Solutions for Class 11 Chemistry Chapter 10 - The s-Block Elements solved by Expert Teachers as per NCERT CBSE textbook guidelines. Ionic radius decreases moving from left to right across a row or period. However within a group non-metallic character decreases from top to bottom.
In a period the non-metallic character increases from left to right. We would like to show you a description here but the site wont allow us. In moving along the period from left to right the MP of these metals first INCREASES to MAXIMUM and the DECREASES regularly towards the end of the period.
Why does metallic bonding peak at about group 6 and decline thereafter. The metallic character of an element can be defined as how readily an atom can lose an electron. A atomic number and therefore charge on the nucleus nuclear or core charge increases b number of valence electrons increases c atomic radius decreases d first ionisation energy increases f electronegativity increases excluding argon g elements on the left are metals elements on.
While moving down in the group from top to bottom the non metallic character decreases. 97 Of The Time Chemotherapy Does not Work. Doctors and pharmaceutical companies make money from it.
Thus C is more non-metallic than Si. Thus among B C N and F non-metallic character decreases in the order. In fact it does the opposite.
All Chapter 10 - The s-Block Elements Exercises Questions with Solutions to help you to revise complete Syllabus and boost your score more in examinations. Metallic character increases as you move down a group because the. C Non-metallic character decreases across a period with increase in atomic number.

How Does The Metallic Character Of Elements Change On Going From Left To Right In A Period Of The Periodic Table

How Does Metallic Character Change When We Move I Across A Period From Left To Right Ii Down A Group

Metallic Character And Non Metallic Character In Periodic Table Chemistry Youtube

Zinc Group Element Chemistry Britannica

Metallic Bond Properties Examples Explanation Britannica

Metallic And Non Metallic Character Periodic Trends Examples Videos
All Periodic Trends In Periodic Table Explained With Image

Gothic Gorget With Pauldrons Medieval Collectibles Pauldron Leather Armor Shoulder Armor
All Periodic Trends In Periodic Table Explained With Image

Periodic Trend Of Metallic Character Video Khan Academy

Parrish Dining Table Dining Table Live Edge Dining Table Table

Ginger Spice Iced Cherry Crown Paint Colors Ginger Spice Spices Crown Paint Colours

Metallic Character Trend On The Periodic Table

Periodic Classification Of Elements Class 10 Notes Science Chapter 5 Learn Cbse Class10sciencenotes Scien Science Notes Study Chemistry Teaching Chemistry

Icse Grade 10 Chemistry Periodic Table Lessons Exercises And Practice Tests Teaching Chemistry Chemistry Classroom Science Chemistry
Metallic Character Of Third Row Elements Meaning Trend Factors Affecting

Castle Clash Troops Google Search Castle Clash Ogre 2d Game Art

Hatshepsut S Golden Beehive Bee Hive Bee Queen Bees

Castle Clash Troops Google Search Castle Clash Ogre 2d Game Art
Comments
Post a Comment